Healthcare White Paper
Reducing the Cost and Risk of HAIs
The Challenge of HAIs for Hospital Leaders
Healthcare-associated infections (HAIs) remain a significant threat to patient safety, financial performance, and hospital reputation. These infections increase patient morbidity, prolong hospital stays, and drive up healthcare costs—costing U.S. hospitals an estimated $28.4 to $45 billion annually.¹ C. difficile infections alone cost hospitals approximately $25,000 per case and contribute to Medicare penalties under the Hospital-Acquired Condition (HAC) Reduction Program.² Under this CMS program, hospitals in the worst-performing quartile for hospital-acquired conditions—including HAIs—face up to a 1% reduction in overall Medicare reimbursements.⁵ Infection data is submitted quarterly through the CDC’s National Healthcare Safety Network (NHSN) and publicly reported via the Care Compare platform.⁶
HAI rates are not just clinical metrics—they’re reputational risks that influence public trust, media coverage, and competitive standing. Infection rates are publicly reported, scrutinized by The Joint Commission, and factored into hospital safety grades and rankings such as those from U.S. News & World Report and The Leapfrog Group.³,⁴ In today’s digital age, infection concerns shared through online reviews or social media can rapidly erode confidence in a hospital’s brand. Persistent outbreaks may lead to unit closures, decreased referrals, and regulatory investigations, creating mounting pressure on hospital leadership to demonstrate a proactive infection control strategy.³,⁴
Evolving the Response: From Manual to Modern
Hospitals have traditionally relied on manual disinfection and hand hygiene to control infections. While essential, these approaches alone leave gaps in coverage and are prone to human error. Ultraviolet (UV) disinfection systems, including UV-C and pulsed xenon, have gained popularity for supplemental disinfection in hospitals across the U.S. Hospitals have adopted UV systems mainly because they reduce labor over manual disinfection. Despite their popularity, the real-world efficacy of UV systems is increasingly questioned. Studies report limitations in pathogen reduction due to UV's reliance on line-of-sight, poor shadow penetration, limited distance of light intensity, and the need for multiple repositionings.³,⁵ In clinical settings, these shortcomings often result in inconsistent disinfection performance, particularly in cluttered or shadowed environments. In contrast, the alternative technology of aerosolized hydrogen peroxide (aHP) is starting to gain traction within hospitals because aHP systems provide uniform room coverage with higher germicidal efficacy and documented pathogen elimination.³,⁶ The following table contrasts UV and aHP systems and explains why aHP systems are a compelling solution for modern hospital disinfection.
Aerosolized Hydrogen Peroxide Comparison with UV Disinfection
|
Feature |
aHP Systems |
UV Systems |
|
Coverage |
Whole-room, including hard-to-reach areas |
Line-of-sight only, distance from UV source limited |
|
Operator Intervention |
One-button operation |
Requires repositioning for full coverage |
|
Treatment Time |
~10 to 15 minutes |
~30 to 60 minutes |
|
Residue |
None |
None |
|
Efficacy vs. C. difficile |
Strong clinical evidence⁴,⁵ |
Mixed performance⁵ |
|
Maintenance & Service |
Typically low |
Typically high-cost bulb replacement |
|
Data Logging |
Often includes time, location, usage, etc. |
Often limited or absent |
The Latest Technology - Automated aHP Systems
Within the last few years, advances in technology have enabled the introduction of automated aerosolized hydrogen peroxide systems to enhance hospital room disinfection. Smart connected IoT technology and digital data platforms can now be combined with aHP technology to enable a leap forward in streamlining hospital infection prevention. Automated aerosolized hydrogen peroxide systems offer the labor-saving advantages of UV systems, combined with the additional benefits of whole room coverage and science-driven clinical evidence of germicidal efficacy against hard-to-kill pathogens. While UV struggles with shadowed areas and requires repeated repositioning, aHP uniformly fills a room with micron-sized droplets, ensuring consistent coverage without the need for operator intervention. Common applications within a hospital environment include the following:
-
Operating room and surgical suite bio-decontamination
-
Terminal cleaning and isolation room disinfection
-
PACU, oncology, and long-term acute care units
-
Outbreak containment and proactive deep cleaning
Treating a hospital room with an automated aHP system requires very little labor and alleviates EVS staff from arduous tasks and chemical exposure. Also, with full room disinfection cycles of less than 15 minutes, a hospital room can be turned over more quickly than alternative methods. A typical whole-room supplemental disinfection process using automated aHP equipment typically includes the following steps:
-
Perform regular cleaning of soiled surfaces and waste, linen replacement, etc.
-
Place the automated aHP system within the room, initiate operation, and leave the room during the countdown time
-
Leave the room closed off for the aHP contact and dissipation time (typically 10-15 minutes) - upon completion, the room is fully disinfected and ready for re-entry
-
Reopen room and move aHP equipment to next room or return to EVS storage
As an added benefit of the latest smart-device technology, connected aHP systems can integrate within data platforms to provide historical data on equipment usage, aHP dosages, room coverage, disinfection times and locations to improve processes and provide auditable records. Some disinfection data platforms will integrate within hospital operational software platforms to provide system-wide hospital data and process management.
An aHP example - Breezy Blue™
Breezy Blue™ is an example of next-generation touchless aHP disinfection. Breezy Blue uses advanced aerosolizing jets that uniformly fill an entire room with micron-sized droplets faster than any other aHP technology. This rapid aHP dissemination allows the room to be filled more quickly and the aHP to dissipate faster, allowing hospital rooms to be fully disinfected with 5-log germicidal efficacy in 10 to 15 minutes. Also, because the disinfection cycles are short, the room does not require sealing or any other time-consuming preparation.
Breezy Blue is used with EPA-registered hydrogen peroxide-based disinfectants such as Breezy HaloSpray™. When used with hospital-grade Breezy HaloSpray, Breezy Blue offers broad germicidal efficacy (bacteria, viruses and fungi), is residue-free, is odorless, and is safe for use around sensitive hospital equipment.¹¹
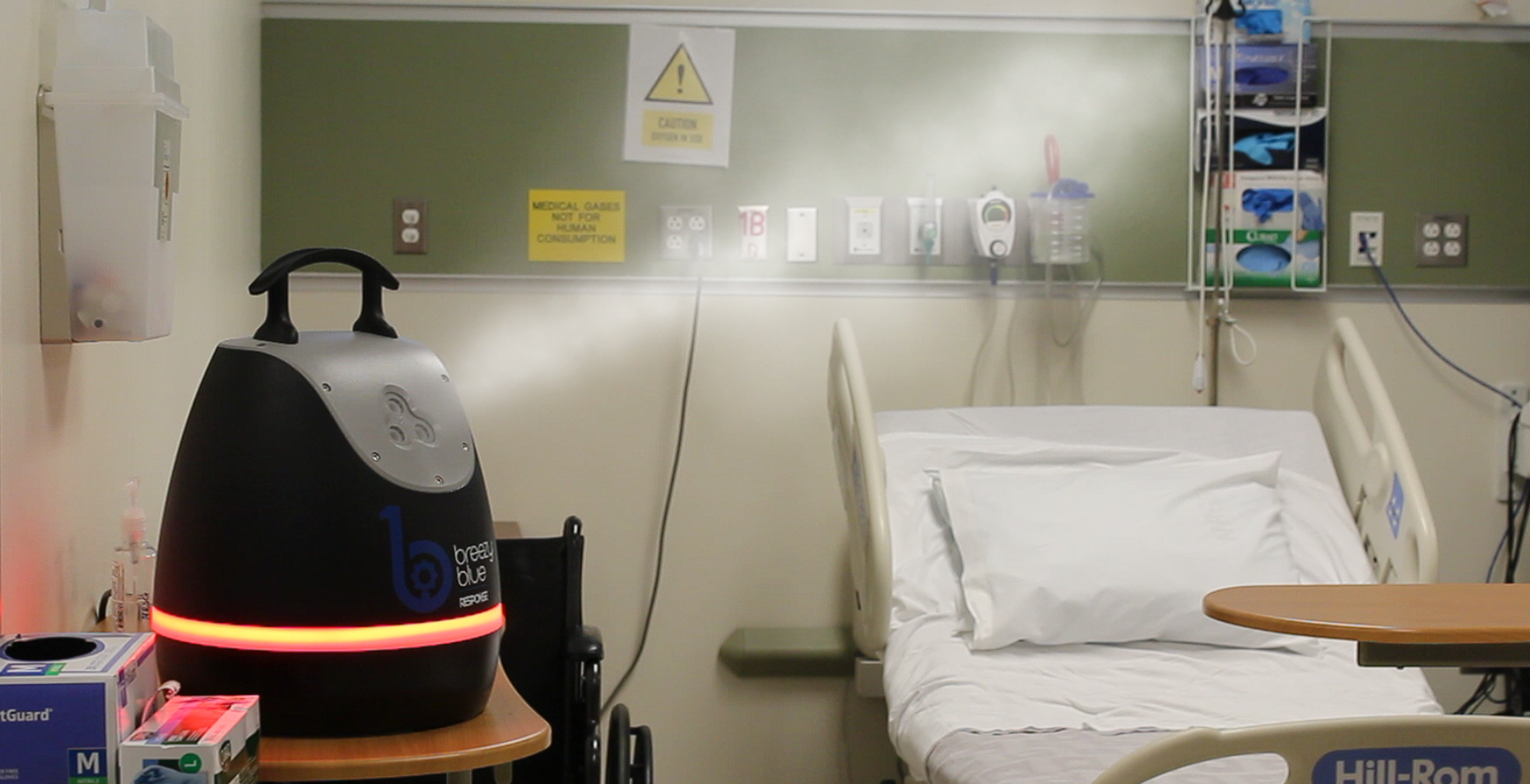
A Connected Hospital example - Breezy Cloud™
Breezy Blue leverages the advantages of the latest technology of smart connected devices integrated with a data platform. Breezy Blue integrates with Breezy Cloud™, a data warehouse with user dashboards for disinfection statistics, to simplify workflows for environmental services (EVS) staff and to provide verified compliance data. All Breezy Blue and Breezy Cloud connectivity is accomplished outside of the hospital IT infrastructure, providing more data segregation and inherent protection of any sensitive hospital data or systems. Breezy Cloud's automatic recording and other features include:
-
Treatment location, time, and dosage
-
Disinfectant and equipment usage statistics
-
Operational validation and status
-
Compliance logs for audits
-
API integration with RTLS and hospital platforms
Breezy Cloud supports hospital quality programs, Joint Commission readiness, and system-wide performance tracking. For example, Breezy Cloud provides exportable logs showing disinfection events by room, treatment durations, and compliance trends over time—useful for both internal audits and CMS reporting.
Conclusion
HAIs pose a critical operational and reputational risk for hospitals. Automated aHP systems offer a leap forward in streamlining hospital room disinfection with traceable logs for audit compliance. For hospital leaders, this represents a strategic opportunity to improve safety, reduce costs, and protect institutional reputation.
Breezy Blue and Breezy Cloud integrate into existing EVS workflows, require minimal training, and can be deployed across departments without infrastructure changes. This is one option for a proven, automated solution to modernize infection prevention with validated results, ease of use, and measurable ROI, including these specific benefits :
Clinical Efficacy: aHP is validated by peer-reviewed studies for its effectiveness against high-risk pathogens.
- AJIC Study: Demonstrated >99.9999% reduction of C. difficile, MRSA, and VRE.⁷
- Automated Disinfection Overview: Supported the superior coverage and efficacy of aHP systems over UV-C.⁸
- SARS-CoV-2 Study: Showed 100% elimination of viral RNA from air and surfaces post-aHP treatment.⁹
- Mold/Fungal Study: Verified elimination of Aspergillus spp. in healthcare settings.¹⁰
Ease of Use: One-button start, visual and auditory status, no manual repositioning, and no PPE required.
Total Coverage: Fills the entire room with uniform micron-sized aHP droplets with dosage options between 4-log and 6-log germicidal efficacy.
Operational Efficiency: Thorough whole-room disinfection can be completed in less than 15 minutes, allowing hospital rooms to be turned over quickly.
Verified Compliance: Breezy Cloud tracks usage, treatment data, and generates audit-ready reports.
References
-
Gidey, K., et al. “Clinical and economic burden of healthcare-associated infections: A prospective cohort study.” PLoS ONE, 2023. https://pmc.ncbi.nlm.nih.gov/articles/PMC9949640/
-
Truitt, C. L., et al. “Evaluation of an aerosolized hydrogen peroxide disinfection system.” AJIC, 2021. https://doi.org/10.1016/j.ajic.2021.11.021
-
The Leapfrog Group. Hospital Safety Grade methodology. https://www.hospitalsafetygrade.org/
-
U.S. News & World Report. Best Hospitals methodology. https://health.usnews.com/health-care/best-hospitals/articles/faq-how-and-why-we-rank-and-rate-hospitals
-
CMS Hospital-Acquired Condition Reduction Program. https://www.cms.gov/medicare/quality/value-based-programs/hospital-acquired-conditions
-
CDC National Healthcare Safety Network (NHSN). https://www.cdc.gov/nhsn/index.html
-
Amodio, E.., et al. “Disinfecting noncritical medical equipment with aerosolized hydrogen peroxide.” AJIC, 2020. https://pubmed.ncbi.nlm.nih.gov/32464292/
-
Otter, J. A., et al. “An overview of automated room disinfection systems.” J Hosp Infect, 2020. https://pmc.ncbi.nlm.nih.gov/articles/PMC7152068/
-
Alnimr, A., et al. “Environmental Deposition of SARS-CoV-2: Role of Aerosolized Hydrogen Peroxide.” Risk Management and Healthcare Policy, 2021. https://doi.org/10.2147/RMHP.S336085
-
Triggiano, F., et al. “No-Touch Automated Disinfection System Based on Hydrogen Peroxide and Ethyl Alcohol Aerosols for Use in Healthcare Environments.” International Journal of Environmental Research and Public Health, 2022. https://pmc.ncbi.nlm.nih.gov/articles/PMC9029184/
👉 Download a pdf copy of this whitepaper here: Reducing the Cost & Risk of HAIs
Calculate Your Return on Investment
Use our form to enter your specific hospital data to calculate your expense reduction and ROI of moving to Breezy Blue within your hospital

